First of all, I want to thank the Graduate School of Geosciences (GSGS) and the steering committee for funding my start-up grant from 01/2018 to 09/2018. The start-up grant provided me the great opportunity to work as a Research Assistant (WHK) and to proceed with the scientific topic by deepening scientific literature- and laboratory work. Thanks to the funding, it was possible to submit a DFG proposal.
Manganese (Mn) is an essential trace element with a complex environmental behaviour due to its various possible oxidation states (-III to +VII). In natural water environments, Mn occurs in a di- (Mn(II)), tri- (Mn(III)) and tetravalent (Mn(IV)) state. However, Mn(III) is concerned as an unstable intermediate undergoing rapid disproportionation to Mn(II) and Mn(IV) through one electron step reactions.
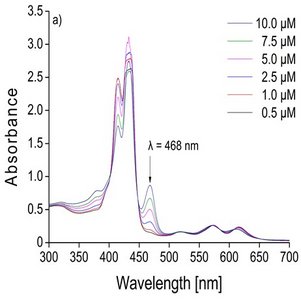
In the laboratory, I was able to adapt a recently devel-oped speciation method (Madison et al. 2011) used in marine chemistry to trace and discriminate Mn(III) at very low concentrations ranging from 0.1 to 10 µM. Speciation of Mn will be achieved using a spectro-photometric (UV-Vis spectrometer) kinetic method which differentiates Mn(II), as well as weak and strong organic complexes of Mn(III). The main chemical principle of the method is the utilisation of a cadmium (Cd) coordinating porphyrin complex which allows a metal substitution reaction between Cd and Mn. The specific Soret band at 468 nm illus-trates the formation of the Mn(III)-porphyrin complex depending on different Mn concentrations (Fig. 1). The main kinetic driven reactions behind the method are as following:
Mn(II) + Cd(II)–porphyrin → Cd(II) + Mn(II)–porphyrin + O2 → Mn(III)–porphyrin
Mn(III) + Cd(II)–porphyrin → Cd(II) + Mn(III)–porphyrin
During the funding period, I performed several experiments in triplicate using topsoil samples (Cambisol, Gleysol and Stagnosol). Serum vials and inert gas were used to simulate appropriate reducing conditions. Aluminium foil was wound around the vials to block ambient light. The samples were shaken for one week on a horizontal shaker to ensure steady mixing. Following this, the liquid phase was collected and the spectrophotometric kinetic method was applied to each solution. The solutions were also checked by flame atomic absorption spectroscopy (F-AAS) to examine the quality of the obtained data. The results of both the spectroscopic method and F-AAS showed that I was able to adapt the method to aqueous soil environments successfully. These results encourage further investigations of soil pore water solutions using a state-of-the-art bioreactor. The bioreactor will give the possibility to analyse soluble Mn(III) in several soil horizons by controlling master variables like, e.g., redox potential (EH) and pH Hence, successful method adaptation will lead to in-situ measurements during field studies. The aim of the research project is to provide a first insight into the occurrence and behaviour of Mn(III) in soil solutions, also concerning the impact of natural organic ligands as potential Mn(III) stabilizing complexes. Neglecting the intermediate Mn(III) species would underestimate the redox capacity of the soluble Mn pool in soil solutions. Furthermore, there might be an impact on topics like breakdown of soil organic matter, degradation of organic contaminants and oxidation of particulate organic carbon to the greenhouse gas carbon dioxide.
References:
Madison, AS, BM Tebo & GW Luther. 2011. Simultaneous determination of soluble manganese(III), manganese(II) and total manganese in natural (pore)waters. Talanta 84: 374–381.
Constantin Lux
PhD student
Institute of Geography, University of Cologne
Thesis: Is manganese(III) a ubiquitous part of the total dissolved manganese in soil solutions?
Supervisor: Professor Dr. Tim Mansfeldt